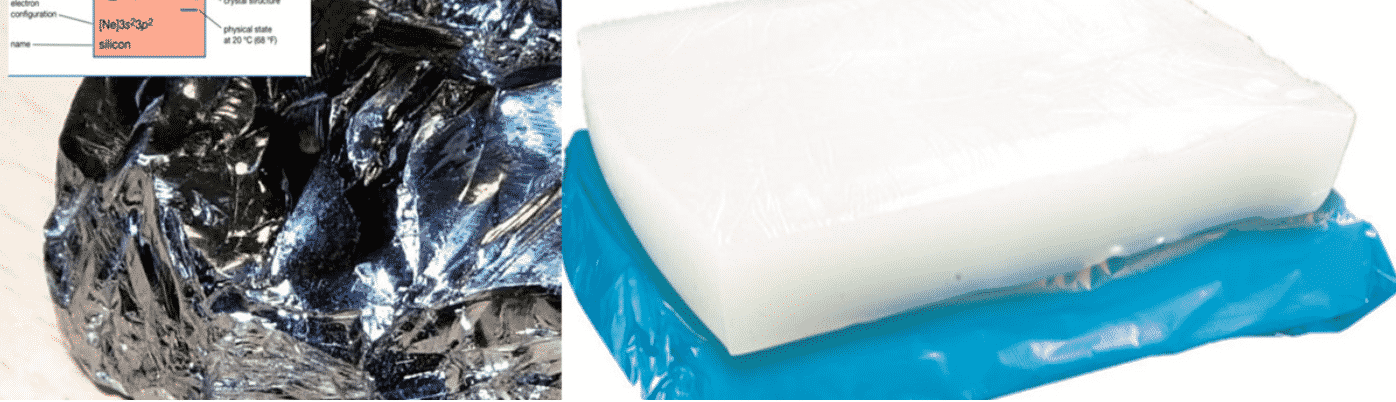
Silicon vs Silicone
What’s the Difference between them?
It’s very easy to mix up the words silicone vs. silicon since the only visible difference is that one of the words has an “e” at the end. Even though they’re spelled so similarly, these two words represent two very different substances.Both materials are widely used, both contain silicon, and they are almost spelled the same, but there are significant differences.
one is an element from the periodic table, while the other is a compound that contains that element. They aren’t the same, any more than saying carbon and fat are the same thing. Silicon is a natural chemical element, Silicon, a non-metallic element, is a semiconductor material that can be used to make semiconductor devices and integrated circuits. It was formerly known as “silicon”.
silicone is a man-made product.Silicone is a polyorganosiloxane with siloxane (—R₂Si—O—SiR₂—) as the structural unit, where R represents organic groups such as methyl and phenyl. This type of polymer has a repeated Si—O bond as the main chain, and the organic group is directly connected to the silicon atom. The general formula is (where n=1-3, m≥2).
Definition and structure
Silicone is a polymer compound composed of siloxane units. The main chain is composed of alternating silicon atoms and oxygen atoms (Si—O—Si), which is similar to the structure of quartz (silicon dioxide), but the side chain is connected to organic groups (such as methyl and phenyl). Its chemical formula can be expressed as [R₂SiO]ₙ, where n represents the number of silicon-oxygen chain segments and m represents the degree of polymerization.
Application classification
The main products include silicone oil, silicone rubber, silicone resin, etc., which are widely used in cosmetics, electronics industry, aerospace and other fields.
In short, silicon is the chemical element Si, while silicone is a synthetic polymer.Clarifying the difference between the two is crucial for industry practitioners and brand communications.
What Is Silicon?
Silicon (Silicon), a non-metallic element in the third period and fourth group of the periodic table, has an element symbol of Si, an atomic number of 14, and a relative atomic mass of 28.086. There are two types of single substances: crystalline and amorphous. Crystalline silicon is blue-gray, with a relative density of 2.32-2.34 g/cm3, a melting point of 1414°C, and a boiling point of 2355°C. Amorphous silicon is a gray-black powder, insoluble in water and hydrogen fluoride solution, but soluble in alkali and a mixture of hydrogen fluoride and nitric acid. Crystalline silicon has obvious conductivity, which is lower than that of metals, and increases with increasing temperature. High-purity silicon doped with trace phosphorus can be used to prepare n-type semiconductors, and doped with trace boron can be used to prepare p-type semiconductors. It is inactive at room temperature and has no obvious effect on air, water, and acid (except hydrofluoric acid and its mixed acid). It can slowly dissolve in concentrated alkaline solution to form soluble silicates and release hydrogen. It can react with halogens to form silicon tetrahalide when heated. It can react with non-metallic elements such as oxygen, carbon, nitrogen, and sulfur at high temperatures. It can also react with metals such as calcium and magnesium to form corresponding metal silicides. It can dissolve in a mixed acid of concentrated nitric acid and hydrofluoric acid to form silicon dioxide, which then dissolves into silicon tetrafluoride.
Silicon is also an extremely common element. In nature, it is usually in the form of complex silicates or silicon dioxide, and is widely present in rocks, gravel, and dust. Silicon ranks eighth in the universe in terms of reserves. It is widely distributed in dust, sand, planets, and planets in various forms of silicon dioxide (silicates) or silicates. The content of silicon in the earth’s crust is second only to oxygen. However, silicon is rare in nature. Natural silicon is only found in skarn-type sulfur and polymetallic deposits in Fujian. It is bright gray-silver white, has a strong metallic luster, and is brittle.
Because of its excellent semiconductor properties, silicon has become the main element for making computer chips and is indispensable in the electronics industry, computer industry, optical fiber communications, and solar energy.

What Is Silicon Used for?
Silicon’s core application areas cover semiconductors, new energy, chemical materials, communication technology and other modern industries. Its unique properties support the development of key areas such as the electronics industry, photovoltaic power generation, and silicone manufacturing.
Silicon quickly and easily forms a chemical bond with the element oxygen (O) so it’s rarely, if ever, observed in nature its pure form. However, it is present in many products and has uses in many industrial applications
Mostly, silicon uses are for a commercial purpose that does not require separation and requires minimum processing of silicon-based natural minerals. Some of the silicon uses include its industrial application in silica sand, clays, and stone. Portland cement uses silicates for stucco and mortar. Mix it with gravel and silica sand, and you have got concrete for walkways, roads, and foundations. Silicon is also useful in producing white-ware ceramics like porcelain. while silicon dioxide is the core material for optical fiber communications.
Silicon carbides, which are silicon compounds, can be helpful as abrasives and materials for high-strength ceramics. Silicon also provides the basis for synthetic polymers known as silicones, including silicone rubber, silicone oil, silicone resin, etc., are widely used in automobile manufacturing (seals), medical equipment (catheters), construction (waterproof materials) and other fields.The late 20th to early 21st century has been coined as Silicon Age, also known as the “Information Age” or “Digital Age.” It is all due to the significant impact of elemental silicon on the economy of the modern world.
Although used in a small portion of semiconductor electronics at less than 10%, the highly purified silicon element is essential to MOS or metal-oxide silicon transistors. Integrated chips also use silicon in the most advanced technology, such as cell phones and computers. For example, Metal-oxide Silicon Field-Effect Transistors or MOSFET is the most popular and successful silicon device. It is probably the most manufactured device in a large number than any other in the history of technology and sciences. Industries such as aluminium-casting, steel refining, and fine chemical producers often use free silicon to make fumed silica. Semiconductors and Electronics Industry:Chip Manufacturing: High-purity silicon is the core substrate of electronic components such as integrated circuits, CPUs, and memory, supporting the operation of digital devices such as computers and smartphones. Applied to semiconductor lasers, LEDs, OLEDs and other fields to meet the needs of power electronic equipment for high-efficiency materials.
New Energy and Sustainable Development
Photovoltaic Power Generation: Solar-grade polysilicon is the core raw material of photovoltaic cells, supporting the large-scale development of the global photovoltaic industry.
Wind Power Storage: Silicone sealants are used for waterproof sealing and component protection of wind turbines to improve equipment durability.
Silicon is also an essential element in biology, although animal physiology only requires traces of silicon in their bodies. However, various ocean species, such as sea sponges and microorganisms like radiolaria and diatoms, are known to secrete silica-based skeletal structures. Silica is also known to deposit in various plant tissues.

What Is Silicone?
Unlike the element silicon, silicone is a synthetic compound made up of siloxanes. Silicone is made of silicon, oxygen, and other elements like hydrogen and carbon. Silicones (there are many) are refers to compounds containing Si-C bonds and at least one organic group directly connected to silicon atoms. Compounds that connect organic groups to silicon atoms through oxygen, sulfur, nitrogen, etc. are also usually considered organic silicon compounds. Among them, polysiloxanes with silicon-oxygen bonds (-Si-O-Si-) as the skeleton are the most numerous, most deeply studied, and most widely used type of organic silicon compounds, accounting for more than 90% of the total usage.
Due to its unique structure, silicone combines the properties of both inorganic and organic materials. It has basic properties such as low surface tension, low viscosity-temperature coefficient, high compressibility, and high gas permeability. It also has excellent properties such as high and low temperature resistance, electrical insulation, oxidation stability, weather resistance, flame retardancy, hydrophobicity, corrosion resistance, non-toxicity, and physiological inertness. It is widely used in aerospace, electronics, construction, transportation, chemical, textile, food, light industry, and medical industries. Silicone is mainly used for sealing, bonding, lubrication, coating, surface activity, demoulding, defoaming, foam suppression, waterproofing, moisture-proofing, and inert filling. With the continuous growth in the number and variety of silicones, the application fields are constantly expanding, forming an important product system that is unique in the chemical new materials industry. Many varieties are indispensable and cannot be replaced by other chemicals.
Organisms also need the participation of silicones in metabolism. Usually, such silicones exist in the form of silicates or silane ethers . Silicones play an important role in various functions of the body and are directly related to the absorption of minerals. The average human body has about seven grams of silicon, which is far more than other important minerals such as iron. Iron and silicon are essential elements for the human body and play a very important role in maintaining normal metabolism.
Organic silicon materials can be divided into: silane coupling agents (organic silicon chemical reagents), bioactive organic silicon, silicone oil (silicone grease, silicone emulsion, silicone surfactant), high temperature vulcanized silicone rubber, liquid silicone rubber, silicone resin, composites, etc. according to their different forms.
According to their different forms, silicone materials can be divided into: silane coupling agents (silicone chemical reagents), silicone oil (silicone grease, silicone emulsion, silicone surfactant), high temperature vulcanized silicone rubber, liquid silicone rubber, silicone resin, composites, etc.
Silane coupling agent
There are generally three ways to apply silane coupling agents:
One is as a surface treatment agent for skeleton materials; the second is to add it to adhesives; the third is to add it directly to polymer materials. From the perspective of giving full play to its effectiveness and reducing costs, the first two methods are better.
The application of silane coupling agents can be roughly summarized into three aspects:
- Used for surface treatment of glass fiber, it can improve the bonding performance of glass fiber and resin, greatly improve the strength, electrical, water resistance, weather resistance and other properties of glass fiber reinforced composite materials, even in wet state, it has a significant effect on improving the mechanical properties of composite materials. The use of silane coupling agents in glass fibers is quite common, and the silane coupling agents used in this aspect account for about 50% of its total consumption, among which the most commonly used varieties are vinyl silane, amino silane, methacryloxy silane, etc.
- Used for filling plastics with inorganic fillers. The filler can be surface treated in advance or added directly to the resin. It can improve the dispersibility and adhesion of the filler in the resin, improve the process performance and improve the mechanical, electrical and weather resistance of the filled plastic (including rubber).
- Used as a tackifier for sealants, adhesives and coatings, it can improve their bonding strength, water resistance, weather resistance and other properties. Silane coupling agents can often solve the problem that some materials have long been unable to bond.
Bioactive silicone
Silicones that can be fully absorbed by organisms are widely found in plants, such as wheat, oats and other cereals. So far, scientists have found that the highest content is verbena (Equisetumarvense). So far, only some developed countries such as France have mastered the extraction technology of high-purity liquid bioabsorbable silicone.
Silicone oil
Silicone oil is a polysiloxane with a chain structure of different polymerization degrees. The most commonly used silicone oil is methyl silicone oil. Silicone oil is generally a colorless (or light yellow), odorless, non-toxic, and non-volatile liquid. Silicone oil is insoluble in water, methanol, glycol and ethoxyethanol, but is miscible with benzene, dimethyl ether, methyl ethyl ketone, carbon tetrachloride or kerosene, and slightly soluble in acetone, dioxane, ethanol and butanol. It has a very small vapor pressure, a high flash point and ignition point, and a low freezing point. As the number of chain segments n changes, the molecular weight increases and the viscosity also increases, so silicone oil can have various viscosities. According to the chemical structure, silicone oil can be divided into methyl silicone oil, ethyl silicone oil, phenyl silicone oil, methyl hydrogen silicone oil, methyl phenyl silicone oil, methyl chlorophenyl silicone oil, methyl ethoxy silicone oil, methyl trifluoropropyl silicone oil, methyl vinyl silicone oil, methyl hydroxy silicone oil, ethyl hydrogen silicone oil, hydroxy hydrogen silicone oil, cyanide silicone oil, etc.; according to the purpose, there are damping silicone oil, diffusion pump silicone oil, hydraulic oil, insulating oil, heat transfer oil, brake oil, etc. Silicone oil has excellent heat resistance, electrical insulation, weather resistance, hydrophobicity, physiological inertness and low surface tension. In addition, it has a low viscosity-temperature coefficient and high compression resistance. Some varieties also have radiation resistance.
Organic silicon emulsion (a form of silicone oil) mainly includes silicone oil fabric softening finishing agent; silicone oil emulsion type defoamer: it is the most widely used and largest amount of organic silicon defoamer.
Silicone rubber - Room temperature vulcanized silicone rubber
Room temperature vulcanized silicone rubber (RTV) is a new type of organic silicon elastomer that came out in the 1960s. The most notable feature of this rubber is that it can be cured in situ at room temperature without heating or pressurization, and it is extremely convenient to use. Therefore, it quickly became an important part of the entire organic silicon product as soon as it came out. Room temperature vulcanized silicone rubber has been widely used as adhesives, sealants, protective coatings, potting and molding materials, and has its uses in all walks of life.
Classification
Room temperature vulcanized silicone rubber can be divided into single-component and two-component room temperature vulcanized silicone rubber according to its packaging method, and can be divided into condensation type and addition type according to the vulcanization mechanism. Therefore, room temperature vulcanized silicone rubber can be divided into three types according to its composition, vulcanization mechanism and use process, namely single-component room temperature vulcanized silicone rubber, two-component condensation type room temperature vulcanized silicone rubber and two-component addition type room temperature vulcanized liquid silicone rubber. These three series of room temperature vulcanized silicone rubber each have their own characteristics: the advantage of single-component room temperature vulcanized silicone rubber is that it is easy to use, but the deep curing speed is difficult; the advantage of two-component room temperature vulcanized silicone rubber is that it does not release heat during curing, has a very small shrinkage rate, does not expand, has no internal stress, and can be cured simultaneously inside and on the surface, and can be deeply vulcanized; the vulcanization time of addition type room temperature vulcanized silicone rubber is mainly determined by temperature, so the vulcanization speed can be controlled by adjusting the temperature.
Single-component room temperature vulcanized silicone rubber
The vulcanization reaction of single-component room temperature vulcanized silicone rubber is to react with moisture in the air to vulcanize into an elastomer. With different chain agents, single-component room temperature vulcanized silicone rubber can be of many varieties such as deacidification type, deoxime type, dealcoholization type, deamine type, deamide type and deketone type. The vulcanization time of single-component room temperature vulcanized silicone rubber depends on the vulcanization system, temperature, humidity and thickness of the silicone rubber layer. Increasing the temperature and humidity of the environment can accelerate the vulcanization process. Under typical environmental conditions, the surface of the silicone rubber can be non-sticky after 15 to 30 minutes, and the adhesive layer with a thickness of 0.3 cm can be cured within one day. The depth and strength of curing will gradually increase in about three weeks.
Single-component room temperature vulcanized silicone rubber has excellent electrical properties and chemical inertness, as well as heat resistance, natural aging resistance, flame resistance, moisture resistance, and breathability. They can maintain elasticity for a long time in the range of -60 to 200 ° C. It does not absorb heat or release heat during curing, has a small shrinkage rate after curing, and has good adhesion to materials. Therefore, it is mainly used as an adhesive and sealant. Other applications include in-situ formed gaskets, protective coatings, and caulking materials. Many one-component silicone rubber adhesives are formulated to exhibit automatic bonding properties to a variety of materials such as most metals, glass, ceramics and concrete. When bonding is difficult, a primer can be applied to the substrate to improve the bonding strength. The primer can be a reactive silane monomer or resin. When they cure on the substrate, they form a modified surface suitable for silicone bonding. Although one-component room temperature vulcanized silicone rubber is easy to use, its vulcanization is dependent on moisture in the atmosphere, which limits the thickness of the vulcanized rubber and can only be used in situations where a thickness of less than 6 mm is required. The vulcanization reaction of one-component room temperature vulcanized silicone rubber is gradually carried out from the surface to the depth. The thicker the glue layer, the slower the curing. When the deep part also needs to be cured quickly, the layered pouring step-by-step vulcanization method can be used. Some glue can be added each time, and then added after vulcanization, which can reduce the total vulcanization time. Adding magnesium oxide can accelerate the vulcanization of the deep glue.
Two-component condensation room temperature vulcanized silicone rubber
The vulcanization reaction of two-component room temperature vulcanized silicone rubber is not initiated by moisture in the air, but by a catalyst. Usually, the rubber and the catalyst are packaged as a component. Curing only begins when the two components are completely mixed together. The curing time of two-component condensation-type room temperature vulcanized silicone rubber mainly depends on the type, amount and temperature of the catalyst. The more catalyst is used, the faster the vulcanization and the shorter the shelf time. At room temperature, the shelf time is generally a few hours. If you want to extend the shelf time of the rubber, you can use the cooling method. It takes about a day for two-component condensation-type room temperature vulcanized silicone rubber to reach full curing at room temperature, but it only takes 1 hour at a temperature of 150°C. The curing speed can be significantly increased by using an accelerator for a synergistic effect.
Two-component room temperature vulcanized silicone rubber can maintain elasticity for a long time in the temperature range of 65 to 250°C, and has excellent electrical properties and chemical stability. It is resistant to water, ozone and weathering. In addition, it is simple to use and has strong process applicability. Therefore, it is widely used as a potting and molding material. After various electronic and electrical components are coated and potted with room temperature vulcanized silicone rubber, they can play a protective role such as moisture-proof (corrosion-proof, shock-proof, etc.). It can improve performance and stability parameters. Two-component room temperature vulcanized silicone rubber is particularly suitable for deep potting materials and has a faster vulcanization time, which is superior to single-component room temperature vulcanized silicone rubber. Two-component room temperature vulcanized silicone rubber has excellent anti-sticking properties after vulcanization, and the shrinkage rate during vulcanization is extremely small. Therefore, it is suitable for making soft molds for casting molds of epoxy resin, polyester resin, polystyrene, polyurethane, vinyl plastic, paraffin, low melting point alloys, etc. In addition, the high simulation performance of two-component room temperature vulcanized silicone rubber can be used to replicate various exquisite patterns on cultural relics. When using two-component room temperature vulcanized silicone rubber, attention should be paid: first weigh the rubber and catalyst separately, and then mix them in proportion. Mixing process The process should be carefully operated to minimize the amount of entrained gas. After the rubber material is mixed (uniform color), the bubbles can be removed by standing or reducing pressure (vacuum degree 700 mmHg). After all the bubbles are discharged, it is placed at room temperature or at a specified temperature for a certain period of time to vulcanize into silicone rubber.
Two-component addition-type room temperature vulcanized silicone rubber
Two-component addition-type room temperature vulcanized silicone rubber is divided into elastic silicone gel and silicone rubber. The former has lower strength and the latter has higher strength. Their vulcanization mechanism is based on the addition reaction (hydrogen silylation reaction) between the vinyl (or propylene) on the end group of the organic silicone raw rubber and the silicon hydrogen group on the crosslinking agent molecule. In this reaction, no by-products are released. Since low molecular weight substances are not released during the crosslinking process, the addition-type room temperature vulcanized silicone rubber does not shrink during the vulcanization process. This type of vulcanized rubber is non-toxic, has high mechanical strength, and has excellent resistance to Hydrolysis stability (even under high-pressure steam), good low compression set, low flammability, deep vulcanization, and vulcanization speed can be controlled by temperature. Therefore, it is a type of silicone rubber that is being vigorously developed at home and abroad.
Addition-type room temperature vulcanized silicone rubber
The packaging method is generally divided into two components, A and B: the catalyst is used as one component; the crosslinking agent is used as another component. High-strength addition-type room temperature vulcanized silicone rubber is an excellent material for molding because of its low linear shrinkage and no release of low molecules during vulcanization. It has been widely used in the machinery industry to mold epoxy resins, polyester resins, polyurethanes, polystyrene, vinyl plastics, paraffin, low melting point alloys, concrete, etc. Using addition-type room temperature vulcanization - High temperature vulcanized silicone rubber
High temperature vulcanized silicone rubber is a high molecular weight (molecular weight is generally 400,000 to 800,000) polysilicone Silicone (i.e. raw rubber) is added with reinforcing fillers and various other additives, and organic peroxide is used as a vulcanizing agent. It is pressurized (molded, extruded, calendered) or injected, and cross-linked into rubber at high temperature. This rubber is generally referred to as silicone rubber.
The reinforcing filler of silicone rubber is various types of white carbon black, which can increase the strength of the vulcanized rubber tenfold. The addition of various additives is mainly to reduce the cost of the rubber, improve the properties of the rubber, and give the vulcanized rubber various special properties such as flame retardant and conductive. - Silicone gel
After vulcanization, this rubber becomes a soft and transparent organic silicone gel, which can maintain elasticity for a long time in the temperature range of -65 to 200°C. It has excellent electrical properties and chemical stability, water resistance, ozone resistance, weather aging resistance, hydrophobicity, moisture resistance, shock resistance, no corrosion, and is physiologically inert, non-toxic, odorless, easy to infuse, can be deeply vulcanized, and has linear shrinkage. With the advantages of low rate and simple operation, silicone gel is widely used as moisture-proof, insulating coating and potting material for electronic components in the electronics industry, and it protects electronic components and assemblies from dust, moisture, shock and insulation. If transparent gel is used to pot electronic components, it can not only protect against shock and water, but also allow the components to be seen and the fault of the components to be detected with a probe, and replaced. The damaged silicone gel can be potted and repaired again. Due to its high purity, easy use and certain elasticity, silicone gel is an ideal internal coating material for transistors and integrated circuits, which can improve the pass rate and reliability of semiconductor devices; silicone gel can also be used as an elastic adhesive for optical instruments. In medicine, silicone gel can be used as an organ implanted in the human body, such as an artificial breast, and used to repair damaged organs. - Foam Silicone rubber
Foam silicone rubber is in liquid state before vulcanization and is suitable for potting materials. Foam silicone rubber is an ideal lightweight packaging material because of its high thermal stability, good thermal insulation, insulation, moisture resistance, and seismic resistance, especially good seismic resistance at high frequencies.
Dow Corning of the United States has developed a flame-retardant room temperature vulcanized foam silicone rubber DC3-6548. This foam silicone rubber is mainly used for fireproof sealing of wires and cables (such as holes in roofs, walls, buildings, etc.). It has very good flame retardant properties, with a limiting oxygen index of 39 (the limiting oxygen index of most plastics is only 20), and a service life of up to 50 years. This flame-retardant room temperature vulcanized foam silicone rubber has been widely used in nuclear power plants, electronic computer centers, offshore oil production equipment and other places with harsh environmental conditions or particularly high fire protection requirements.
Silicone resin
Silicone resin is a highly cross-linked network The polyorganosiloxane with a quaternary structure is usually prepared by hydrolyzing various mixtures of methyltrichlorosilane, dimethyldichlorosilane, phenyltrichlorosilane, diphenyldichlorosilane or methylphenyldichlorosilane at a relatively low temperature in the presence of an organic solvent such as toluene to obtain an acidic hydrolyzate. The initial product of the hydrolysis is a mixture of cyclic, linear and cross-linked polymers, which usually also contains a considerable number of hydroxyl groups. The hydrolyzate is washed with water to remove the acid, and the neutral initial polycondensate is thermally oxidized in the air or further polycondensed in the presence of a catalyst to finally form a highly cross-linked three-dimensional network structure.
Silicone resin is a thermosetting plastic, and one of its most outstanding properties is its excellent thermal oxidative stability. After heating at 250°C for 24 hours, the weight loss of silicone resin is only 2-8%. Another outstanding property of silicone resin is its excellent electrical insulation properties, which can maintain its electrical insulation properties over a wide temperature and frequency range. Maintain its good insulation properties.
In view of the above characteristics, silicone resin is mainly used as insulating varnish (including varnish, enamel, color paint, impregnation paint, etc.) to impregnate H-class motors and transformer coils, and to impregnate glass cloth, glass cloth and asbestos cloth to make motor casings, electrical insulation windings, etc. Large-area mica sheet insulation materials can be made by bonding mica with silicone insulating varnish, which is used as the main insulation of high-voltage motors. In addition, silicone resin can also be used as heat-resistant and weather-resistant anti-corrosion coatings, metal protective coatings, waterproof and moisture-proof coatings for construction projects, release agents, adhesives, and secondary processing into silicone plastics, which are used in the electronics, electrical and defense industries as semiconductor packaging materials and insulation materials for electronic and electrical parts.
Silicone resin can be roughly divided into several categories according to its main use and cross-linking method, such as silicone insulating varnish, silicone coating, silicone plastic and silicone adhesive.
The
中文(简体) – 检测到的语言英语中文(简体)德语
英语中文(简体)法语
Yǒujī guī chǎnpǐn de jīběn jiégòu dānyuán shì yóu guī-yǎng liàn jiégòuchéng de, cè liàn zé tōngguò guī yuánzǐ yǔ qítā gè zhǒng yǒujī jī tuán xiānglián. Yīncǐ, zài yǒujī guī chǎnpǐn de jiégòu zhōng jì hányǒu”yǒujī jī tuán”, yòu hányǒu”wújī jiégòu”, zhè zhǒng tèshū de zǔchéng hé fēnzǐ jiégòu shǐ tā jí yǒujīwù de tèxìng yǔ wújī wù de gōngnéng yú yīshēn. Yǔ qítā gāo fēnzǐ cáiliào xiāng bǐ, yǒujī guī chǎnpǐn de zuì túchū xìngnéng shì: Nài wēn tèxìng yǒujī guī chǎnpǐn shì yǐ guī-yǎng (Si-O) jiàn wéi zhǔ liàn jiē gòu de,C-C jiàn de jiàn néng wéi 82.6 Qiān kǎ/kè fēnzǐ,Si-O jiàn de jiàn néng zài yǒujī guī zhōng wèi 121 qiān kǎ/kè fēnzǐ, suǒyǐ yǒujī guī chǎnpǐn de rè wěndìng xìng gāo, gāowēn xià (huò fúshè zhàoshè) fēnzǐ de huàxuéjiàn bùduànliè, bù fēnjiě. Yǒujī guī bùdàn kě nài gāowēn, érqiě yě nài dīwēn, kě zài yīgè hěn kuān de wēndù fànwéi nèi shǐyòng. Wúlùn shì huàxué xìngnéng háishì wùlǐ jīxiè xìngnéng, suí wēndù de biànhuà dōu hěn xiǎo. Nàihòu xìng yǒujī guī chǎnpǐn de zhǔ liàn wèi-Si-O-, wúshuāng jiàn cúnzài, yīncǐ bùyì bèi zǐwài guāng hé chòuyǎng suǒ fēnjiě. Yǒujī guī jùyǒu bǐ qítā gāo fēnzǐ cáiliào gèng hǎo de rè wěndìng xìng yǐjí nài fú zhào hé nàihòu nénglì. Yǒujī guī zhōng zìrán huánjìng xià de shǐyòng shòumìng kě dá jǐ shí nián. Diànqì juéyuán xìngnéng yǒujī guī chǎnpǐn dōu jùyǒu liánghǎo de diàn juéyuán xìngnéng, qí jiè diàn sǔnhào, nài diànyā, nài diànhú, nài diàn yūn, tǐjī diànzǔ xìshù hé biǎomiàn diànzǔ xìshù děng jūn zài juéyuán cáiliào zhōng mínglièqiánmáo, érqiě tāmen de diànqì xìngnéng shòu wēndù hé pínlǜ de yǐngxiǎng hěn xiǎo. Yīncǐ, tāmen shì yī zhǒng wěndìng de diàn juéyuán cáiliào, bèi guǎngfàn yìngyòng yú diànzǐ, diànqì gōngyè shàng. Yǒujī guī chúle jùyǒu yōuliáng de nài rè xìng wài, hái jùyǒu yōuyì de jù shuǐxìng, zhè shì diànqì shèbèi zài shī tài tiáojiàn xià shǐyòng jùyǒu gāo kěkào xìng de bǎozhàng. Shēngwù tèxìng shēngwù huóxìng yǒujī guī shì réntǐ bìxū de yī zhǒng de yíngyǎngsù. Yǒujī guī shì gòuchéng réntǐ zǔzhī hé cānyù xīnchéndàixiè de zhòngyào yuánsù. Cún yú réntǐ de měi yīgè xìbāo dāngzhōng, zuòwéi xìbāo gòujiàn de zhīchēng, tóngshí bāngzhù qítā zhòngyào wùzhí rú měi, lín, gài děng xīshōu. Réntǐ zhǐ néng tōngguò shíwù bù duàn huòdé yǒujī guī. Kēxuéjiāmen rènwéi, yǒujī guī zhǔyào yǐ sān zhǒng xíngshì cúnzài yú réntǐ zhōng: (Yī) kěróngxìng yǒujī guī, zhàn zhòngliàng de 10% (èr) bǎi fēn zhī sānshí cúnzài yú gè zhǒng xìbāo jīzhì (sān)60%yòng lái héchéng dànbáizhí zhè shuōmíng wǒmen měitiān suǒ xū de yǒujī guī shì xiāngdāng gāo. Rúguǒ yào bǎochí 5 nián,10 nián shènzhì yúshì 30 nián de niánqīng chéngdù, měitiān shè rù yǒujī guī 20-30 háokè de yǒujī guī yóuwéi zhòngyào. Dī biǎomiàn zhānglì hé dī biǎomiàn néng yǒujī guī de zhǔ liàn shífēn róushùn, qí fèn zǐ jiān de zuòyòng lì bǐ tàn qīng huàhéwù yào ruò dé duō, yīncǐ, bǐ tóng fēnzǐ liàng de tàn qīng huàhéwù niándù dī, biǎomiàn zhānglì ruò, biǎomiàn néng xiǎo, chéng mó nénglì qiáng. Zhè zhǒng dī biǎomiàn zhāng lì hé dī biǎomiàn néng shì tā huòdé duō fāngmiàn yìngyòng de zhǔyào yuányīn: Shūshuǐ, xiāo pào, pàomò wěndìng, fáng zhān, rùnhuá, shàng guāng děng gè xiàng yōuyì xìngnéng.
展开
949 / 5,000
The basic structural unit of silicone products is composed of silicon-oxygen chain segments, and the side chains are connected to various other organic groups through silicon atoms. Therefore, the structure of silicone products contains both “organic groups” and “inorganic structures”. This special composition and molecular structure make it a combination of the characteristics of organic matter and the functions of inorganic matter. Compared with other polymer materials, the most outstanding performance of silicone products is: Heat resistance Silicon products are based on silicon-oxygen (Si-O) bonds as the main chain structure. The bond energy of C-C bonds is 82.6 kcal/g molecule, and the bond energy of Si-O bonds in silicone is 121 kcal/g molecule. Therefore, silicone products have high thermal stability, and the chemical bonds of molecules do not break or decompose at high temperatures (or radiation exposure). Silicone is not only resistant to high temperatures, but also to low temperatures, and can be used in a wide temperature range. Both chemical properties and physical and mechanical properties change very little with temperature. Weather resistance The main chain of silicone products is -Si-O-, and there is no double bond, so it is not easily decomposed by ultraviolet light and ozone. Silicone has better thermal stability, radiation resistance and weather resistance than other polymer materials. The service life of silicone in natural environment can reach several decades. Electrical insulation performance Silicone products have good electrical insulation performance. Their dielectric loss, voltage resistance, arc resistance, corona resistance, volume resistivity and surface resistivity are among the best in insulating materials, and their electrical properties are little affected by temperature and frequency. Therefore, they are a stable electrical insulation material and are widely used in the electronics and electrical industries. In addition to excellent heat resistance, silicone also has excellent water repellency, which is a guarantee for the high reliability of electrical equipment under wet conditions. Biological properties Biologically active silicone is a nutrient necessary for the human body. Silicone is an important element that constitutes human tissue and participates in metabolism. It exists in every cell of the human body, serves as a support for cell construction, and helps absorb other important substances such as magnesium, phosphorus, calcium, etc. The human body can only continuously obtain silicone through food. Scientists believe that silicone exists in the human body in three main forms: (i) Soluble silicone, accounting for 10% of the weight (ii) 30% exists in various cell matrices (iii) 60% is used to synthesize proteins This shows that the silicone we need every day is quite high. If you want to stay young for 5, 10 or even 30 years, it is particularly important to consume 20-30 mg of silicone every day. Low surface tension and low surface energy The main chain of silicone is very flexible, and the intermolecular force is much weaker than that of hydrocarbons. Therefore, it has lower viscosity, weaker surface tension, smaller surface energy and stronger film-forming ability than hydrocarbons of the same molecular weight. This low surface tension and low surface energy are the main reasons for its many applications: hydrophobic, defoaming, foam stability, anti-sticking, lubrication, glazing and other excellent properties.
The Feature of silicone
The basic structural unit of silicone products is composed of silicon-oxygen chain segments, and the side chains are connected to various other organic groups through silicon atoms. Therefore, the structure of silicone products contains both “organic groups” and “inorganic structures”. This special composition and molecular structure make it a combination of the characteristics of organic matter and the functions of inorganic matter. Compared with other polymer materials, the most outstanding performance of silicone products is:
Heat resistance
Silicon products are based on silicon-oxygen (Si-O) bonds as the main chain structure. The bond energy of C-C bonds is 82.6 kcal/g molecule, and the bond energy of Si-O bonds in silicone is 121 kcal/g molecule. Therefore, silicone products have high thermal stability, and the chemical bonds of molecules do not break or decompose at high temperatures (or radiation exposure). Silicone is not only resistant to high temperatures, but also to low temperatures, and can be used in a wide temperature range. Both chemical properties and physical and mechanical properties change very little with temperature.
Weather resistance
The main chain of silicone products is -Si-O-, and there is no double bond, so it is not easily decomposed by ultraviolet light and ozone. Silicone has better thermal stability, radiation resistance and weather resistance than other polymer materials. The service life of silicone in natural environment can reach several decades.
Electrical insulation performance
Silicone products have good electrical insulation performance. Their dielectric loss, voltage resistance, arc resistance, corona resistance, volume resistivity and surface resistivity are among the best in insulating materials, and their electrical properties are little affected by temperature and frequency. Therefore, they are a stable electrical insulation material and are widely used in the electronics and electrical industries. In addition to excellent heat resistance, silicone also has excellent water repellency, which is a guarantee for the high reliability of electrical equipment under wet conditions.
Biological properties
Biologically active silicone is a nutrient necessary for the human body. Silicone is an important element that constitutes human tissue and participates in metabolism. It exists in every cell of the human body, serves as a support for cell construction, and helps absorb other important substances such as magnesium, phosphorus, calcium, etc. The human body can only continuously obtain silicone through food.
Scientists believe that silicone exists in the human body in three main forms:
(i) Soluble silicone, accounting for 10% of the weight
(ii) 30% exists in various cell matrices
(iii) 60% is used to synthesize proteins This shows that the silicone we need every day is quite high.
If you want to stay young for 5, 10 or even 30 years, it is particularly important to consume 20-30 mg of silicone every day.
Low surface tension and low surface energy
The main chain of silicone is very flexible, and the intermolecular force is much weaker than that of hydrocarbons. Therefore, it has lower viscosity, weaker surface tension, smaller surface energy and stronger film-forming ability than hydrocarbons of the same molecular weight. This low surface tension and low surface energy are the main reasons for its many applications: hydrophobic, defoaming, foam stability, anti-sticking, lubrication, glazing and other excellent properties.
Silicone has a low toxicity and high heat resistance. This makes it a wonderful compound for use in sealants for watertight containers like fish tanks as well as plumbing pipes. The food Grade Silicone material meets FDA and EU requirements for food contact and safety.
The food grade silicone material is soft and comfortable, environmentally friendly, non-toxic, and degradable.They are also,Lead,Reach,BPA,PVC Free.

What Is Silicone Used for?
Silicones are used in many products, from automobile gaskets to electronics coatings to teeth molds. Your ice tray is probably made from them, as are your kitchen utensils and firestops. Over 400,000 tons of silicones were produced in 1991, and the production numbers are huge to this day.
Aside from the properties mentioned above, silicones also exhibit a number of interesting properties which makes them highly sought after. They have a low chemical reactivity, repel microbial growth, have a low toxicity and don’t stick to some substrates, but stick very well to others (such as glass).
electrical for insulation:Composite insulators: Composite insulators made of silicone materials (especially silicone rubber) account for more than 70% of UHV transmission lines due to their strong weather resistance, excellent resistance to pollution flashover and ice flashover, and are the main application direction of the power industry.
Cable accessories and anti-pollution flashover coatings: used to improve the insulation and stability of Electrical equipment:Composite insulators made of silicone materials (especially silicone rubber) account for more than 70% of UHV transmission lines due to their strong weather resistance, excellent resistance to pollution flashover and ice flashover, and are the main application direction of the power industry.
Cable accessories and anti-pollution flashover coatings: used to improve the insulation and stability of electrical equipment.
electronic for coatings:Potting and thermal conductive materials: Used for potting protection of semiconductor components such as LEDs and IGBTs to improve the stability and heat dissipation performance of devices.
Electromagnetic shielding and three-proof paint: Provide physical protection in the manufacture of precision electronic equipment.
Household items such as sealants and cooking utensils, etc
Automobiles :Battery sealing and noise reduction : Organic silicone is used for bonding, sealing and heat conduction of automotive battery parts, while improving the noise reduction effect in the car. Tire and headlight sealing : Improve the sealing and durability of vehicle components.
Seals in airplanes
Keyboard pads in office machines
Tooth impression moulds and other medicine and dentistry industries:As food-grade mold materials (such as chocolate molds, cake molds), and the replication of precision parts, high-temperature resistant silicone stirring spoons, baking tray brushes and other tools are safe and easy to clean, insulation pads, teapot mats, etc., protect the desktop from high temperature damage; anti-slip mats fix items to prevent sliding, silicone foot pads, seat cushions, etc. provide a comfortable experience and adapt to the human body curve design
Coatings in paper and textiles :Building sealing and coatings: Silicone-modified acrylic resin is used for exterior wall coatings to improve weather resistance.
Textile auxiliaries: Silicone oil is widely used in textile processing as a softener and lubricant.
Daily chemical products: As an additive to personal care products such as shampoo and skin care products, it improves the product texture.
New energy industry
Photovoltaic module sealing : Neutral one-component silicone sealant and two-component silicone structural sealant are used for the frame sealing of photovoltaic modules, with high bonding strength and weather resistance.
Wind turbine blade treatment : A small amount of silicone products are used for surface treatment or epoxy resin modification of wind turbine blades.
Medical industry
Biomedical materials : Silicone materials are used in the manufacture of medical devices due to their good biocompatibility and high temperature resistance.
Nuclear power and other industrial applications
Nuclear island facility sealing : Silicone materials are used for sealing non-core facilities in nuclear power plants.
Flexible electronics and aerospace : Provide bonding and protection in flexible displays and spacecraft components.

Who and Why would you like to know the difference of silicon vs silicone?
Semiconductor/electronic engineers, concerned about silicon, focus on the purity and crystal structure of silicon for electronics and semiconductors; medical/kitchenware/maternal and infant product managers focus on silicone for sealing, medical, kitchenware; safety and certification.
Wide range of uses: from chips to baking mats, from automotive seals to medical implants
Different regulatory environments: Silicon is non-toxic and risk-free; silicone polymers need to manage residual catalysts
Brand marketing and communication personnel are sensitive to communication: misuse of terms can easily lead to consumer doubts
Material selection engineers: need to identify the differences in thermal/mechanical/chemical properties between the two.
When and Where will you use the silicon or silicone?
Product naming/packaging, Silicon chip manufacturing, solar cells, semiconductor wafers, etc., high-purity, crystalline materials, Silicon sealants, kitchenware, medical devices, gaskets, rubber molds, wire insulation, three-proof coatings, etc. must accurately describe the material to avoid misunderstanding;
Certification application stage: Silicon products need to be carefully applied, silicon does not need to be
Market communication: Those who are concerned about environmental protection and sustainability need to understand the material science clearly.
How to produce silicon?
Silicon purification is a technical system for removing impurities from industrial silicon through physical, chemical and metallurgical methods. The metallurgical method controls the alloy composition (such as copper-silicon alloy) and combines it with a directional solidification process, using temperature gradients and traveling magnetic fields to reduce metal inclusions. The chemical method uses boiling hydrochloric acid and hydrofluoric acid for step-by-step pickling, and then uses induction plasma flame treatment to volatilize impurities, and the silicon purity can be increased from 3N to 6N. Physical distillation technology is based on the difference in boiling points between silicon and impurity elements, and realizes multi-stage distillation separation in a vacuum environment, which reduces energy consumption compared to the Siemens method. The modified Siemens method separates chloride mixtures through a distillation tower, and uses an anti-disproportionation reaction to convert silicon tetrachloride, establishing a closed circulation system to improve efficiency.
Metallurgical purification
The hypereutectic system is formed by melting crude silicon through copper-silicon alloy (silicon atoms account for 60-95at.%) or aluminum-silicon alloy (silicon atoms account for 55-95at.%), and the Ga-In-Sn alloy liquid is forced to cool, and the primary silicon is sequentially solidified under a stable interface. The traveling wave magnetic field accelerates the convection of the melt, and the pressure and liquid level are adjusted synchronously, so that the metal impurities are enriched at the tail of the ingot, and the silicon ingot with improved purity is obtained after removal. The process reduces metal inclusions by 70%, and the subsequent pickling process is shortened by 50%.
Chemical pickling and plasma treatment
After the industrial silicon is crushed to 300 mesh, the impurities are leached out with boiling hydrochloric acid (concentration 30%) and room temperature hydrofluoric acid (concentration 40%) in turn. The silicon powder after pickling melts the surface in a 30-40kW induction plasma flame, and the hydrogen carrier gas (flow rate 5-150L/min) carries volatile impurities, and rapid cooling causes internal impurities to gather on the surface. After two cycles of treatment, the silicon loss is controlled within 2%, and the purity reaches 6N level.
Physical distillation and rectification technology
In a vacuum or argon environment, the impure silicon is heated to 1414℃, and the boiling point difference between phosphorus (boiling point 280℃), arsenic (614℃) and silicon (2355℃) is used to vaporize and separate in stages. When the pressure is controlled at 10^-3Pa, the silicon evaporation rate is increased by 3 times, and different fractions are collected through a multi-stage condenser. This technology has an efficiency of 98% for the treatment of boron-containing waste and is suitable for the recycling of solar cell raw materials.
Improved Siemens process
Through silicon tetrachloride distillation and purification, a five-tower series system is used to remove BCl3 with a boiling point of 12.1℃ and PCl3 with a boiling point of 76℃. The anti-disproportionation reaction system converts SiCl4 into SiHCl3, and the conversion rate exceeds 85% when the reaction temperature is controlled at 500℃. The distillation tower is designed with stainless steel corrugated packing, which can achieve the separation of 99.9999% pure chloride.
Segregation effect and evaporation effect
According to the segregation coefficient formula C_s=k_eff×C_L, impurities such as copper (k=4×10^-4) and iron (k=8×10^-6) are concentrated at the tail of the silicon ingot during directional solidification. When the solidification rate is controlled to 1mm/min, the impurity concentration at the tail is significantly increased, and the resistivity distribution uniformity is improved after removal. The vacuum evaporation step (pressure 0.1Pa) makes the evaporation rate of aluminum and calcium impurities reach 2g/(cm²·h), and the CO gas generated by the graphite crucible is used to reduce the metal oxide.
Laboratory purification method
Silicon dioxide is reduced by magnesium to produce crude silicon (reaction formula: SiO₂+2Mg→2MgO+Si), and the residual magnesium (reaction rate 0.5g/min) and magnesium silicide are subsequently dissolved with hydrochloric acid, and silicon powder with a purity of 99% is obtained after filtration. Industrial-grade purification generates crude silicon through carbon thermal reduction (1500-2000℃), and hydrogen reduction after chlorination distillation obtains electronic-grade silicon.
How to produce silicone?
The key to silicone rubber production lies in the conversion of raw materials. First, silicon is extracted from quartz sand. The main raw materials include silane compounds such as dimethyldichlorosilane and silicon tetrachloride, which gradually form polysiloxane after hydrolysis and condensation reactions. This step is the basis of silica gel production and directly determines the performance and quality of subsequent products.
During the hydrolysis and condensation reaction, first, dimethyldichlorosilane ((CH₃)₂SiCl₂) undergoes hydrolysis in water to generate silicon-oxygen bond (Si-O-Si) polymers. The specific reaction formula is:
(CH₃)₂SiCl₂ + H₂O → (CH₃)₂Si(OH)₂ + HCl
Then, the hydroxyl groups (Si-OH) in these products undergo further condensation to form linear or network-structured polysiloxanes. The reaction formula is:
(CH₃)₂Si(OH)₂ + (CH₃)₂Si(OH)₂ → (CH₃)₂Si-O-Si(CH₃)₂ + H₂O
This series of reactions constitutes the core chemical process of silicone production. Hydrolysis and condensation reactions are the basis of silicone production. After polymerization, different forms are formed, such as liquid, elastomer (LSR, HTV), RTV, etc.
How much of silicon VS silicone ?
Silicon: The high-temperature production and purification costs are extremely high, and it is mainly used in high-end electronics;
Silicone: The production and modification costs are relatively low, but catalysts, fillers and cross-linking are required; the cost of a single piece is low, but the high-temperature vulcanization and catalyst costs are relatively high.
How to choose and control silicon VS silicone?
If you need semiconductor properties, choose Silicon.It need control purity, crystal defects, resistivity testing.
If you need soft, environmentally friendly, durable, heat-resistant, and non-electrical materials, choose Silicone.It need control hanging residual catalyst, hardness, temperature resistance, stretching, aging testing.
How to clean silicon VS silicone?
Silicon wafers: need to be cleaned in a clean room and passivated with DI water;
Silicone products: cleaned with neutral detergent, and need to be thoroughly dried after high temperature use.
More FAQ Silicon VS silicone
1: Are Silicone and Silicone the same thing?
No! The former is an element used in semiconductors; the latter is a synthetic polymer used in kitchenware, sealants, medical treatment, etc.
It is a polymer, but the chemical chain is Si-O-Si instead of a carbon chain, so it has a long life and high temperature resistance, and is closer to high-performance elastic materials
3: Will silicone produce particles like plastic?
The structure of silicone is stable and it is not easy to mechanically decompose to form microplastics, but extreme high temperatures may cause trace oxidation products to be released.
4: How to identify whether kitchenware contains Silicone?
Check whether the packaging is marked with “100% Food Grade Silicone”, whether there is an FDA/LFGB logo and a third-party report.
5: Can silicone products be recycled?
As a thermosetting material, it is difficult to recycle, but environmental protection goals can be achieved through chemical recycling or extending the service life.
Conclusion
The difference between silicon and silicone
So, the difference between the two should be evident by now. They’re both useful for the world economy, but one is a naturally occurring chemical element while the other is a man-made polymer.
Hope that clears things out a bit, but if you have any other questions, feel free to contact us!
ZSR Group has rich experience in producing Silicone products with FDA or LFGB Approved standard.We have the FDA register list number is 3011147430.
Any Silicone products or Silicone project need technical support, you could buy custom Silicone products at ZSR Group.

Technical Related
About Author: Z.S.R International Group
Z.S.R International Group(Hong Kong) co., Limited, is a one-stop supplier for molded silicone products and silicone products molding solution provider in the consumer products field. We offer OEM services from silicone product design to Silicone products contract manufacturing. We have the capability for custom silicone tooling, LSR(Liquid silicone Rubber) molded silicone products, solid silicone molded products, molded silicone multi-colored products. We also can custom molded silicone, custom molded LSR, custom molded dripping injection dispensing(co-injection) silicone multi-colored products.